Configurable Quality Management & Compliance Solutions
Built on a Validated Platform According to FDA Regulations
for Life Changing Industries
AI-Powered Configurable Cloud QMS that Adapts to your Processes and Boosts Efficiencies. Validated According to FDA Regulations and Computer System Validation Guidelines. Stay in Compliance & Move Comfortably into the Future.
About
Cloudtheapp
Introducing Cloudtheapp: AI-Powered Configurability for Cloud-Based Validated Regulatory Compliance Platform & Solutions.
AI-Powered Configurability: Cloudtheapp offers unparalleled configurability, driven by advanced AI technology. Users can easily adapt and customize their solutions using our intuitive graphical interface, eliminating the need for extensive coding.
Cloud-Based: Operating on a Software as a Service (SaaS) model, Cloudtheapp and Amazon AWS manage infrastructure, security updates, maintenance, and all related aspects.
Validated: Our platform adheres to FDA Computer System Validation Guidelines and Good Documentation Practices (GDP). We provide a comprehensive validation package for each platform update, including all necessary documents and artifacts for compliance.
Regulatory: Cloudtheapp complies with FDA 21CFR part 820 (QMSR), Life Sciences cGMP, ISO 13485, ISO 9001, and ISO 22001 standards.
With AI-Powered Configurability, Cloudtheapp revolutionizes compliance processes, enabling organizations to navigate regulatory requirements with greater agility and efficiency.
Watch a
2-min video


-
AI-Powered
Configurability - Isolated Cloud Architecture
- Life Sciences
- PaaS / SaaS
- Application Store
- Stages
AI-Powered
Configurability
At Cloudtheapp, we’ve redefined configurability with advanced AI technology. Our platform offers users unparalleled flexibility through innovative features like Cloudtheapp Thunder and Drag/Drop Designer, empowering them to adapt and customize processes effortlessly. Say goodbye to extensive coding
With Cloudtheapp, you can streamline workflows and boost productivity without the hassle. Our intuitive user interface ensures seamless navigation and optimal performance across all devices, providing a truly exceptional user experience.
Isolated
Cloud Architecture
Our platform is cloud native. Designed for the cloud from inception to ensure unlimited scalability and optimum performance. We leveraged Amazon AWS, the proven leading cloud infrastructure, to guarantee dependability and peace of mind.
Life Sciences
We have the features Life Science companies need and have come to expect; such as field history (Audit Trail), transaction history tracking, electronic signatures (e-sig), forced authentication (on routing a record or saving it), and much more.
PaaS / SaaS
We are a 100% SaaS company with multitenant architecture. This means your system will be up and ready to use seconds after signup, giving instantaneous time to value. Internet access and a standard browser is all that is needed client-side so there is nothing to install. SaaS reduces your IT costs and headaches. Enjoy frequent updates (both system and application specific) at no additional cost. Signup is subscription based with credit card payment. Your system will be up seconds after signup. Multi-tenant simplifies working with suppliers and other external parties without having to manage the extended security if you were to give them access to your network.
Application Store
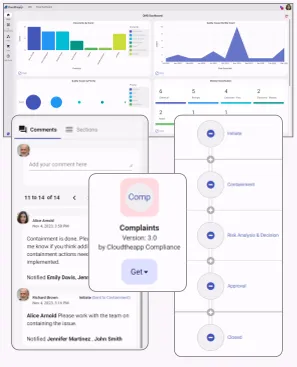
Our Application Store has many applications (both free and paid) ready to deploy with the click of a button, including a full EQMS suite. You decide what to deploy to minimize clutter and avoid confusing your users with applications they don’t use. You can use the application as is, or customize every detail of the design. You can also optionally upload new or modified applications you designed to our store for other companies to use.
Stages
Create unlimited isolated stages (dev, qa, prod etc…) to hold your applications within few seconds. For instance you can design the application initially in a dev stage, then test it in a qa stage, and only go to production when ready. Transfering applications from one stage to another is a 5 second process simply by clicking on the application, then selecting which stage to copy it to.
Enterprise Quality Management System
ISO 9001 | ISO 13485
Our EQMS was built by a team of experts that possess over three decades of industry experience. The main goal was to come up with an Enterprise QMS and Compliance Solutions that remedy all pitfalls of other competing systems. We made collaborating with suppliers, auditors, consumers, or any other external party possible and instant, unlike other systems where you have to have additional disintegrated systems installed or use tedious back and forth emails. Our EQMS builders allow you extend usage of the ready-made applications to build and automate all of your other processes and have them all integrate with each other in hours and without having to write a single line of code.

Customer Success
If you can dream it, you can do it, with Cloudtheapp!
























Features
-
Download
Applications -
Design
Applications - Connect with Suppliers
-
Design Processes
& Workflows -
Single Sign-On
(SSO) - Mobile QR Submission
- Integration API
- Flexible Plans
- Analytics
Download Applications
Deploy free/paid Applications with the Cloudtheapp Application store. You decide which ready-made applications to deploy for your team and platform. Applications are deployed with a simple click and are up and running instantly.
Design Applications
With our state of the art highly intuitive graphical interface builders, you can simply create a new application or modify the design of the ready-made ones. Your applications can be designed by an expert business user instead of a programmer.
Connect with Suppliers
Establish connections using a single account with external parties such as suppliers, auditors, or customers directly within the system. This makes it easier to track, standardize, document, and report on such communication. It also allows configuring workflow steps where a record may be sent directly to a contact from another company to update and send records back to them seamlessly.
Design Processes & Workflows
Consider each of your business processes. Digitalize them using our powerful design interface to directly create the corresponding workflows, forms, and fields. Easily link them to related products or reference them in Document control and other modules. Determine the interaction required in each step, what person or role must be involved, and what path the record must follow. This will facilitate team collaboration and simplify process training for new employees.
Single Sign-On (SSO)
Single Sign-On is available using SAML 2.0. Setup integration with your identity providers (IDPs) such as Microsoft Azure Active Directory to make the login a seamless experience and to ensure centralized administration for users.
Mobile QR Submission
Place system generated QR Codes on Products and in Service Locations, and allow external users like end product consumers to feed your process with information. For example, a product consumer can publicly submit a complaint by scanning a code on the product using a mobile device and without requiring a license.
Integration API
Our Integrations Tool allows businesses to externally communicate with Cloudtheapp platform to implement integration scenarios by putting or getting data to or from Cloudtheapp. Implement integration scenarios with other systems, such as automatic document creation, synchronizing with ERP or CRM, and much more.
Flexible Plans
Cloudtheapp will never force you to pay for applications you don’t want or to pay for a fixed number of users on all the applications you use. You can subscribe to the platform only with no applications if you only wish to create your own, or pick which applications you subscribe to, set a different number of users as needed for each individual application. If you opted for a monthly subscription, change the number of users, add/remove applications at any time in a self-service fashion, or otherwise enjoy the stability and discounts of an annual commitment.
Analytics
Our advanced analytics solution allows decision makers to easily visualize data and have better insight for everyday decisions. Create graphs and visualizations, organize them in dashboards, and share them.
Industries we Serve
Built for Life Changing Industries
Recent Posted Blog
Transforming Operational Excellence: Introducing the Operational Checklists Application
In our continuous pursuit of innovation in quality, compliance, and operational management solutions, Cloudtheapp Inc. proudly presents the Operational Checklists Application. This cutting-edge addition to
🚀 Cloudtheapp is proud to sponsor the Pharmaceutical Development Conference (PDC 2025)
We’re pleased to sponsor the upcoming Pharmaceutical Development Conference (PDC-2025), taking place on September 9, 2025, at the Holiday Inn, Warwick Farm, NSW – Sydney,
Cloudtheapp Launches Advanced Clinical Trials Solution Featuring AI-Driven Configurability
We are proud to announce the release of Cloudtheapp’s Clinical Trials Management Solution, a groundbreaking digital platform engineered to transform how life sciences organizations manage